Every week, Harvard Medical School neuro-oncologist Annie Hsieh treats patients with gliomas — the most common type of brain cancer, including the deadliest, glioblastoma.
After Hsieh’s neurosurgeon colleagues remove a glioma surgically, it often looks like none of the cancer is left behind, she says. Radiation and other treatments may follow. Yet gliomas tend to come back, not just at the original site but in distant parts of the brain, threatening neurological harm and, in some cases, death.
What happens in the brain to encourage these tumors to regrow there, while only rarely appearing in other parts of the body? The question has stumped scientists for decades and made gliomas one of the hardest-to-treat cancers. It’s also a mystery that physician-scientist Hsieh has long wanted to solve.
Now, she and HMS collaborators have filled in a piece of the puzzle by providing the first look at the types of neurons in the brain that connect to gliomas.
The team’s findings were reported Wednesday in PNAS.
Profiling the identities and properties of such glioma-innervating neurons in mice provides new insights into what drives these cancers’ formation and spread in the brain. The findings can also help researchers devise new treatment strategies to stop these tumors from coming back.
“This is a first step that provides a visual explanation for why the tumors can be everywhere in the brain,” said Hsieh, first author of the study and HMS instructor in neurology at Mass General Hospital. “We can now see where the connected neurons originate, study how they integrate with gliomas, and look for opportunities to interrupt growth.”
“It’s fascinating how the neural network functions and how these super-scary tumors integrate with and infiltrate the entire nervous system.”
Annie Hsieh, neuro-oncologist
The study overcomes a long-standing obstacle to visualizing and analyzing the neurons that link with gliomas and demonstrates a way to advance the study of interactions between tumors and the nervous system more broadly.
Hsieh conducted the work when she was a research fellow in neurobiology in the lab of Bernardo Sabatini and in cell biology in the lab of Marcia Haigis in the Blavatnik Institute at HMS. Haigis and Sabatini are co-senior authors of the study.
How gliomas hack the network
Gliomas arise from glia, cells that perform essential functions in sculpting and maintaining neural circuits. Scientists already knew that neurons form synapses onto glioma cells, but they couldn’t see where the other ends of those neurons (the cell bodies) are in the brain. That obscured the neurons’ identities.
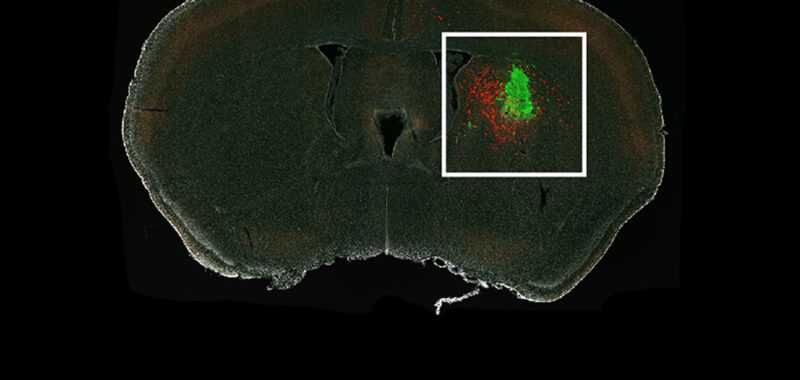
Hsieh and team successfully traced the glioma-innervating neurons back to their sources using a rabies virus engineered to infect only specific cells of interest and to light up those cells when it gets in. The virus travels from the tumor cell back through the neuron that connects to it.
The researchers injected human glioma cells into the brains of mice and waited for neurons to connect with the tumors. They then applied the rabies virus to light up cells of interest. Soon, they had a picture illuminating the mouse brains showing all the glowing neurons that led to the glioma.
The maps revealed that the gliomas hook into existing patterns of neuronal wiring.
“The wires are already there; the gliomas just connect to them,” Hsieh said. “They hijack what’s already in place rather than forming their own arbitrary connections.”
And those neurons originate from across the brain, the researchers observed.
“They come all the way from the interior part of brain to go to the tumor,” Hsieh said. “It’s fascinating how the neural network functions and how these super-scary tumors integrate with and infiltrate the entire nervous system.”
Unmasking neurons’ secret identities
The team found that most of the glioma-innervating neurons extending from the far reaches of the brain are the type that makes glutamate, a major brain chemical that excites neurons. This finding aligns with previous observations that neuronal excitation stimulates glioma growth, and that neuron-glioma communication involves glutamate.
Subsets of the far-reaching glioma-innervating neurons, though, showed signs that they make both glutamate and another chemical called GABA, which inhibits neuronal activity. In some brain areas, glioma-innervating neurons from near the tumor site appeared to be largely GABAergic.
The results suggest that neurons that interact with glioma cells are more diverse than currently appreciated. The implications of this for tumor growth and spread are not yet known.
“We see that the tumor is connected to everywhere. Whether these connections provide a path for them to go everywhere is something we need to study,” Hsieh said.
The team probed the electrical properties of the glioma-innervating neurons and found certain differences between them and similar neurons in brains without glioma. Such variations between normal and glioma-innervating neurons or between neuron-neuron and neuron-glioma interactions offer valuable clues to researchers like Hsieh, who seek ways to intervene in cancerous processes while preserving normal function.
The need to develop glioma treatments is urgent, Hsieh said. Researchers have tried to treat gliomas with drugs that work for other types of cancers, but most of them have failed, she noted.
“By unraveling the drivers of glioma-neuron interactions and identifying unique mechanisms, we can explore strategies to interrupt them, potentially stopping the tumors in their tracks and preventing their return,” Hsieh said.
Although she knows it will be many years before discoveries made in the lab translate into therapies for her patients with glioma and others around the world, Hsieh remains optimistic that these latest insights can help move the field forward.
“It’s not close to the clinic yet,” she said, “but it’s one inch forward.”
Additional authors include Sanika Ganesh, Tomasz Kula, Madiha Irshad, Emily A. Ferenczi, Wengang Wang, Yi-Ching Chen, Song-Hua Hu, Zongyu Li, and Shakchhi Joshi.
This work was supported the National Institutes of Health (including National Cancer Institute award K12CA090354), Howard Hughes r Institute, Lubin Family Foundation Scholar Award, American Academy of Neurology, Burroughs Wellcome Fund, Ludwig Center at HMS, and Glenn Foundation for Medical Research. Confocal images were acquired at the Core for Imaging Technology & Education at HMS, and fluorescence in situ hybridization was performed by the Neurobiology Imaging Facility at HMS.
Haigis received research funding from Agilent Technologies and ReFuel Bio; serves on the scientific advisory boards of Alixia, Minovia Therapeutics, and MitoQ; is on the editorial boards of Cell Metabolism and Molecular Cell; and is a consultant and founder of ReFuel Bio.
Source link